23+ Calculating Cell Potential
Web I have measured the cell potential between a number of metals eg. Web When the concentrations in the two compartments are the opposite of the initial concentrations ie 10 M Zn 2 and 10 10 6 M Cu 2 Q 10 10 6 and the cell potential will be reduced to 092 V.

Doubt Solutions Maths Science Cbse Ncert Iit Jee Neet
Calculate the standard cell potential of a voltaic cell that uses the AgAg and SnSn 2 half-cell reactions.

. Web So that would be positive 54 volts so positive 54 plus 166 plus positive 166 volts. Web So this is one form of the equation that relates the standard cell potential right the standard cell potential E zero to the equilibrium constant K. The Variation of E cell with Log Q for a ZnCu Cell.
This is the standard electrode potential for the reaction Ni 2 aq 2e. The more positive value the more likely the substance is to be reduced so obviously 34 is more positive than -76. Electrochemical cells convert chemical energy to electrical energy and vice versa.
Initially log Q 0 and the voltage of the cell is greater than E cell. Web B Using the value given for Ecell and the calculated value of E anode we can calculate the standard potential for the reduction of Ni 2 to Ni from Equation 2042. Web Calculating the Cell Potential.
This electrochemistry video contains. So we can use one of the equations we talked about in the last video that relates to. Web Solution.
Calculating Standard Cell Potentials. Ni Pb Al Cu and Zn by constructing a galvanic cell and then used a voltmeter to measure the potential. The first step is to determine the cell reaction and total cell potential.
Ecell VCuVsolution VsolutionVZn 1634 1634 E c e l l V C u V s o l u t i o n V s. Web So positive 54 plus 166. Ecell Ecathode Eanode 027V Eocathhode 055V E cathode 028V.
Web About Cell Potential Calculator Formula The cell potential calculator uses a simple formula to calculate the potential difference between the cathode and anode of an. If we are reducing zinc 2 to solid zinc the standard reduction potential turns out to be -76 volts. T he net reaction of a voltaic cell constructed from a standard zinc electrode and a standard copper electrode is obtained by adding the two.
All right now that weve found the standard cell potential we can calculate the equilibrium constant. So the standard potential for the cell so E zero cell is equal to 54 plus 166 which is equal to 220 volts. Plus positive 166 volts.
Web This chemistry video tutorial explains how to calculate the standard cell potential of a galvanic cell and an electrolytic cell. Review Galvanic Cell Example Problem for the method to find cell potential of a galvanic cell For this reaction to be galvanic the cadmium reaction must be the oxidation reactionCd Cd 2 2 e-E 0. Web Table 1731 provides a listing of standard electrode potentials for a selection of half-reactions in numerical order and a more extensive listing is given in Standard Electrode.
Web Calculate cell potentials and predict redox spontaneity using standard electrode potentials Unlike the spontaneous oxidation of copper by aqueous silver I ions described in. Web The Nernst equation allows us to determine the spontaneous direction of any redox reaction under any reaction conditions from values of the relevant standard electrode potentials. So the standard potential for the cell so e zero cell was equal to 54 plus 166 which is equal to 220 volts Now that we found the standard cell potential we can calculate the equilibrium constant.
Web The Relationship between Cell Potential Gibbs Energy. Now if I want to calculate the theoretical potential if I have understood this correctly I need to look at a standard reduction potential table and compare the two. The total amount of.
Web If we are reducing copper 2 to solid copper the standard reduction potential is 34 volts. Web If we expand the above expression we see that the cell potential. We can write this.
In order for the cell to be galvanic E 0 cell 0.

Cell Potential Ck 12 Foundation

Ncert Exemplar Class 12 Chemistry Solutions Chapter 3 Electrochemistry

Redox Potentials Of Small Inorganic Radicals And Hexa Aquo Complexes Of First Row Transition Metals In Water A Dft Study Based On The Grand Canonical Ensemble The Journal Of Physical Chemistry A

Accurate Standard Hydrogen Electrode Potential And Applications To The Redox Potentials Of Vitamin C And Nad Nadh The Journal Of Physical Chemistry A

Question Video Calculating The Standard Cell Potential For A Gold Nickel Cell Nagwa
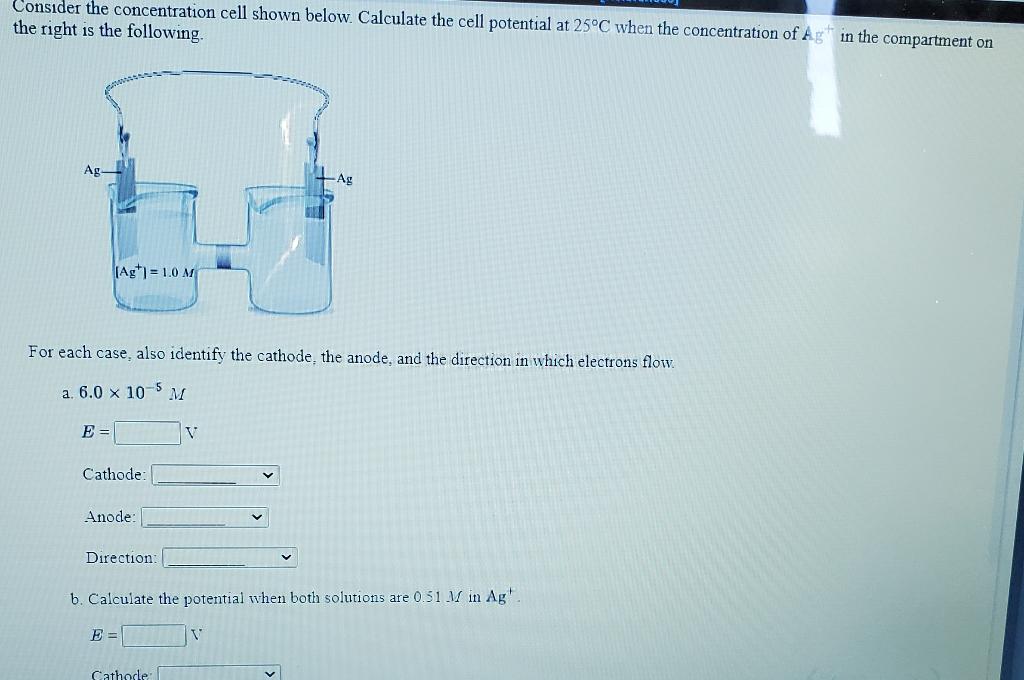
Solved Calculate The Cell Potential At 25 Degrees C When Chegg Com

What Is The Potential Of A Half Cell Consisting Of Zinc Electrode In 0 01 M Znso4 Solution Of 25 Oc E O 0 763v

Calculate The Standard Cell Potentials Of Galvanic Cell In Which The Following Reactions Take Place I 2cr S 3cd 2 Aq 2cr 3 Aq 3cd Ii Fe 2 Aq Ag

How To Find The Cell Potential Ecell Under Standard Conditions Youtube

Accurate Standard Hydrogen Electrode Potential And Applications To The Redox Potentials Of Vitamin C And Nad Nadh The Journal Of Physical Chemistry A
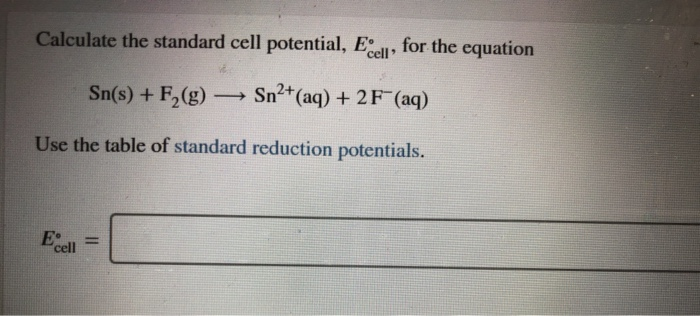
Solved Calculate The Standard Cell Potential El For The Chegg Com
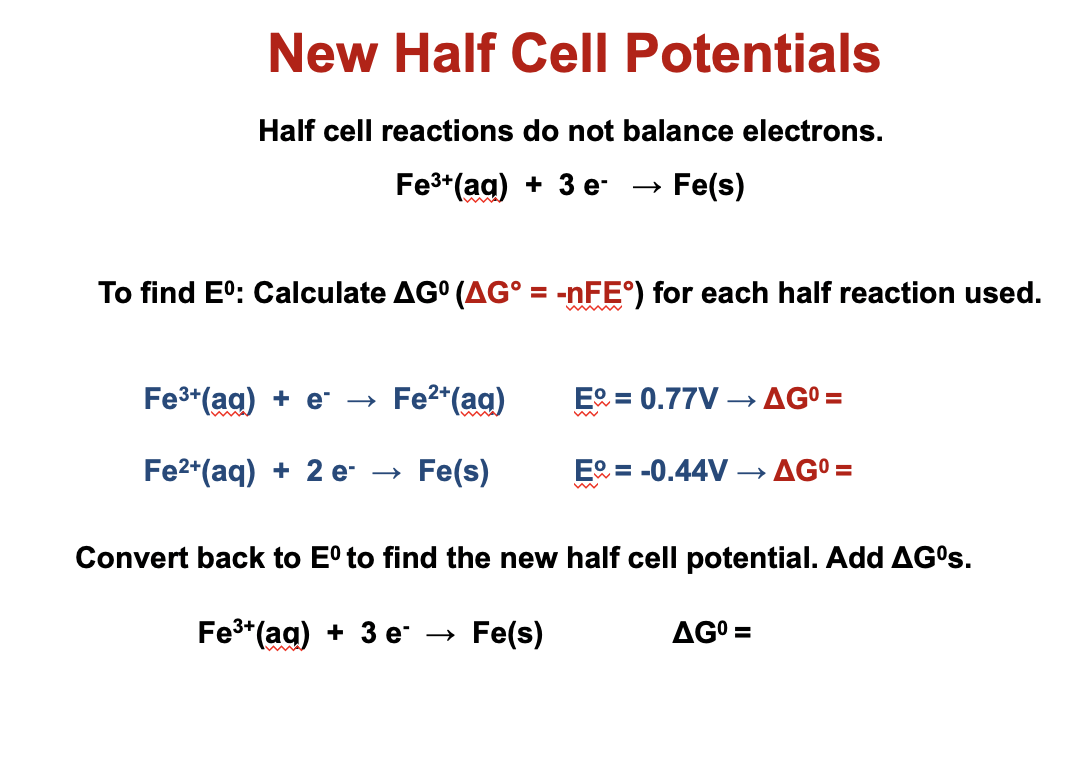
Solved New Half Cell Potentials Half Cell Reactions Do Not Chegg Com
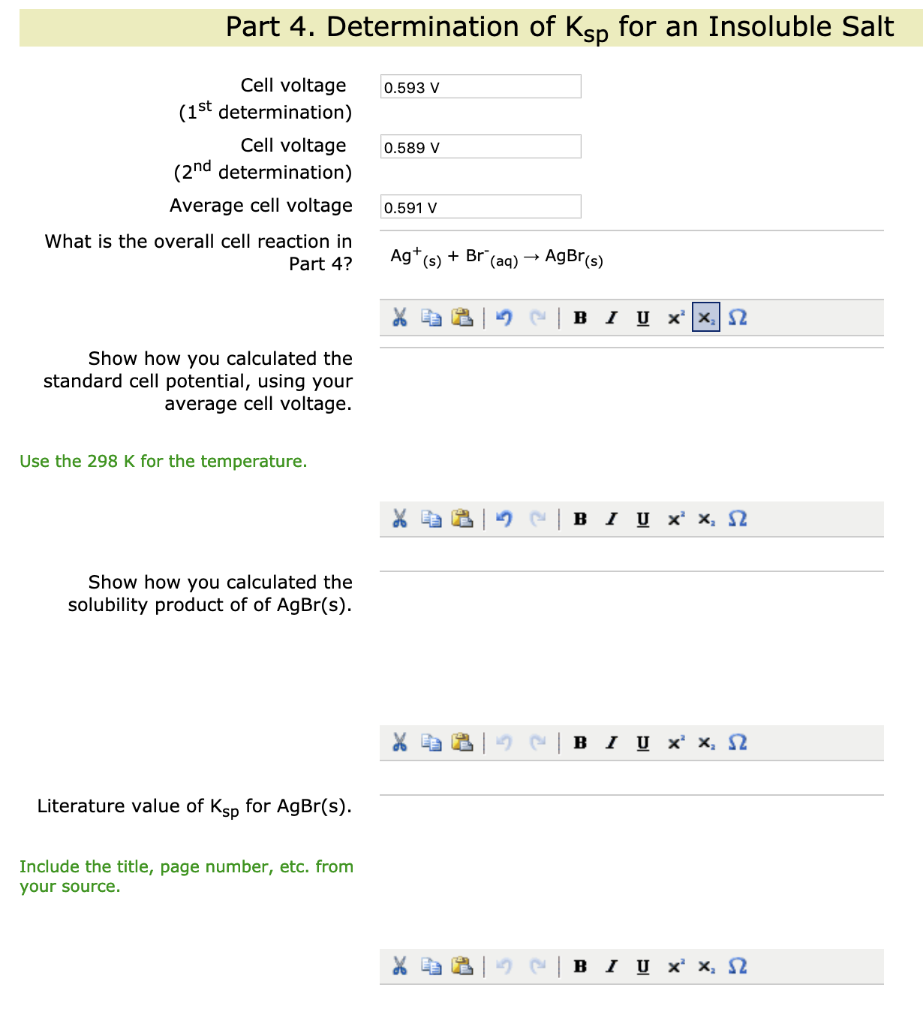
Solved Show How You Calculated The Standard Cell Potential Chegg Com

Calculating Standard Cell Potentials Ck 12 Foundation

Cell Potential Problems Electrochemistry Youtube

How To Find The Cell Potential Step By Step Explanation Tutor Hotline Youtube

19 1 Calculating Cell Potential Hl Youtube